
If you want to reproduce the whole article If you are the author of this article, you do not need to request permission to reproduce figuresĪnd diagrams provided correct acknowledgement is given. Provided correct acknowledgement is given. If you are an author contributing to an RSC publication, you do not need to request permission To request permission to reproduce material from this article, please go to the CV studies of 6 suggested its potential application as a reducing agent, which was further proved via the conversion of Tip–PCl 2 to trimeric (Tip) 3P 3 ( 17), and cAAC P–Cl (cAAC = cyclic alkyl(amino)carbene) to (cAAC) 2P 2 ( 18) and 4, utilizing 6 as a stoichiometric reducing agent.įluorescent organo-antimony compounds as precursors for syntheses of redox-active trimeric and dimeric alkali metal antimonides: an insight into electron transfer reduction processesĮ. The existence of 1˙ − was proved using electron paramagnetic resonance (EPR) spectroscopy in solution. 5 undergoes oligomerization in the solid state to produce 6. Cyclic voltammetry (CV) studies of 1 in THF showed possible two electron reduction, suggesting the in situ generation of the corresponding radical-anion intermediate 1˙ − and its subsequent conversion to the monomeric intermediate (Tip) 2Sb − ( 5) upon further reduction. All the reported compounds have been characterized via NMR, UV-vis, mass spectrometry, and single-crystal X-ray diffraction analysis. Interestingly, the precursors 1 and 4 have been observed to be highly luminescent, emitting green light under short-wavelength UV radiation. The Lewis acidic character of 19 has been unambiguously proved via treatment with Lewis bases to produce the corresponding adducts 20 and 21. Additional reactivity studies involving 1 and AgNTf 2 (Tf = trifluoromethanesulfonyl) afforded the corresponding antimony cation (Tip) 2Sb +NTf 2 − ( 19). In this report, different aspects of the various reducing agents ] used have been studied, correlating the experimental observations with previous reports. Changing the reducing agent from KC 8 to a different alkali metal resulted in the solid-state isolation of corresponding stable dimeric alkali metal antimonides with the general formula (M = Li ( 14), Na ( 15), Cs ( 16)).
The two-electron reduction of 1 and 4 afforded the novel trinuclear antimonide cluster ( 6). Such an arrangement helps explain the periodicity and periodic trends observed across the elements of the periodic table.(Tip) 2SbCl ( 1, Tip = 2,4,6-triisopropylphenyl) has been utilized as a precursor for the synthesis of the distibane (Tip) 4Sb 2 ( 4) via one-electron reduction using KC 8.

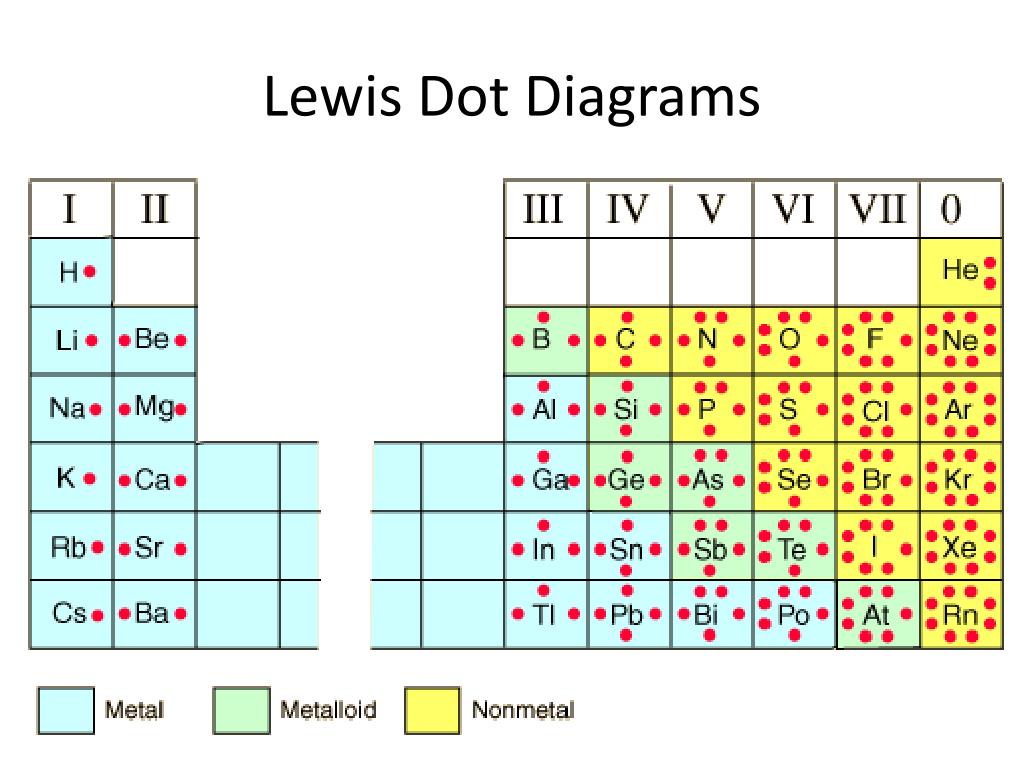
The N shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. The M shell contains 3s, 3p, and 3d, and can carry 18 electrons. The K shell contains a 1s subshell hence it can carry 2 electrons, the L shell has 2s and 2p, and can carry 8 electrons. This decides the electron capacity of the shells. The maximum electrons that can be carried by the sub-shell S is 2, by P is 6, by D is 10, and the F sub-shell can carry 14. Each shell and subshell have a limitation on the amount of electrons that it can carry. The subshells have a distinct shape and configuration, in which the electrons move freely. They stand for sharp (S), principal (P), diffuse (D), and fundamental (F). The shells are labeled K, L, M, N, and so on, from the innermost to the outermost shell.Įach shell has subshells that are named for the type of emission lines produced from different states of angular momentum. This model has been widely accepted, and according to it, each atom has shells, which further have subshells. It involves the specific arrangement of electrons in shells and sub-shells of Bohr’s atomic model. The concept of electronic configuration has replaced the older concept of valency and valence electrons. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.


 0 kommentar(er)
0 kommentar(er)
